Summary
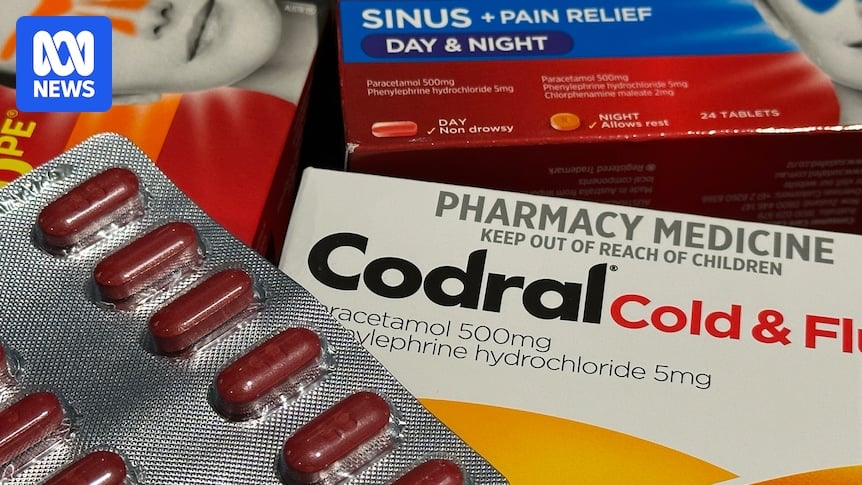
A class action lawsuit has been filed in Australia against Johnson & Johnson, accusing the company of misleading consumers by selling cold and flu medications with phenylephrine, an ineffective oral nasal congestion ingredient.
Products like Codral PE and Sudafed PE replaced pseudoephedrine with phenylephrine in 2006 after stricter regulations.
Lawyers argue consumers were “duped” into buying ineffective products at premium prices.
If successful, buyers since 2005 could be compensated. The TGA is monitoring FDA findings but has no current plans to review oral phenylephrine’s effectiveness.
Why the shit won’t TGA take FDA’s findings into account? There is no significant genetic difference between the populations… Am I missing something here?
It’s normal for these types of agencies to make their own judgments, they shouldn’t assume the FDA knows better. Population and genetic differences shouldn’t matter and won’t be a factor.