- cross-posted to:
- mdma_assisted_therapy
- cross-posted to:
- mdma_assisted_therapy
TL;DR: MDMA is highly effective for PTSD. 90 Participants received either MDMA or placebo plus 3 preparatory and 9 integrative therapy sessions. The MDMA group had significantly lower scores on clinical PTSD surveys. There were few adverse effects and no abuse potential.
Abstract:
Post-traumatic stress disorder (PTSD) presents a major public health problem for which currently available treatments are modestly effective. We report the findings of a randomized, double-blind, placebo-controlled, multi-site phase 3 clinical trial to test the efficacy and safety of MDMA-assisted therapy for the treatment of patients with severe PTSD, including those with common comorbidities such as dissociation, depression, a history of alcohol and substance use disorders, and childhood trauma.
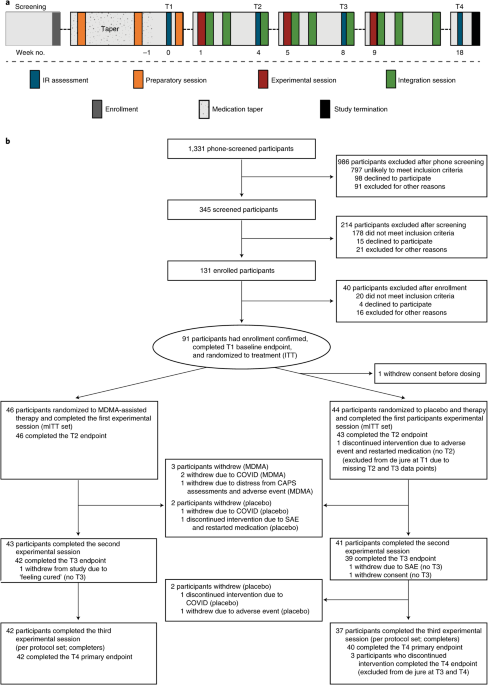
After psychiatric medication washout, participants (n = 90) were randomized 1:1 to receive manualized therapy with MDMA or with placebo, combined with three preparatory and nine integrative therapy sessions. PTSD symptoms, measured with the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5, the primary endpoint), and functional impairment, measured with the Sheehan Disability Scale (SDS, the secondary endpoint) were assessed at baseline and at 2 months after the last experimental session. Adverse events and suicidality were tracked throughout the study.
MDMA was found to induce significant and robust attenuation in CAPS-5 score compared with placebo (P < 0.0001, d = 0.91) and to significantly decrease the SDS total score (P = 0.0116, d = 0.43). The mean change in CAPS-5 scores in participants completing treatment was −24.4 (s.d. 11.6) in the MDMA group and −13.9 (s.d. 11.5) in the placebo group.
MDMA did not induce adverse events of abuse potential, suicidality or QT prolongation. These data indicate that, compared with manualized therapy with inactive placebo, MDMA-assisted therapy is highly efficacious in individuals with severe PTSD, and treatment is safe and well-tolerated, even in those with comorbidities. We conclude that MDMA-assisted therapy represents a potential breakthrough treatment that merits expedited clinical evaluation.
So the MDMA therapy worked. Both groups improved due to therapeutic intervention, but the MDMA group clearly improved a lot more.
One problem, though, and I say this as someone who has taken MDMA a few times: You would not mistake the experience of being on a placebo for the genuine experience of being on molly. They do go into this somewhat under Methods:
An inactive placebo with therapy was utilized as the comparator to isolate the efficacy of the MDMA itself. Although low-dose MDMA improved blinding in phase 2 studies, it led to decreased effectiveness compared with an inactive placebo in a PTSD population, making it easier to detect a difference between the active and comparator groups [15]. The use of inactive placebo also allows for uncontaminated comparison of safety data between groups. Therefore, an inactive placebo was determined in partnership with the FDA as a more conservative statistical comparison, and the study utilized observer-blinded efficacy assessments to minimize bias in efficacy measurements.
Meaning they wanted their two groups to be a) low-dose MDMA vs b) clinical-dose MDMA; but–for reasons I’m honestly not clear on from the above text–they decided to do a) placebo vs. b) clinical-dose MDMA. They claim they use “observer-blinded efficacy assessments” but I just don’t find it plausible that either the observer or the patient would fail to figure out which group they were in after the first dose.
So, that control problem aside, this is a pretty rigorous study and the results are pretty clear.
Couldn’t agree more, and that’s something that future studies will have to address. It’s really hard to truly do double-blind research for psychedelics.