The Food and Drug Administration announced Friday it had broadened the approval of the FluMist nasal spray to become the first “self-administered” influenza vaccine — though a delay in the change means the vaccine will not be available to ship to homes until next year’s flu season at the earliest.
“Today’s approval of the first influenza vaccine for self- or caregiver-administration provides a new option for receiving a safe and effective seasonal influenza vaccine potentially with greater convenience, flexibility and accessibility,” Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in a statement.
The FluMist vaccine, manufactured by AstraZeneca, had previously been approved back in 2003 to be given by health care providers similar to other flu shots. Now the vaccinemaker has approval to sell FluMist to adults for use at home on themselves or to administer to their children.


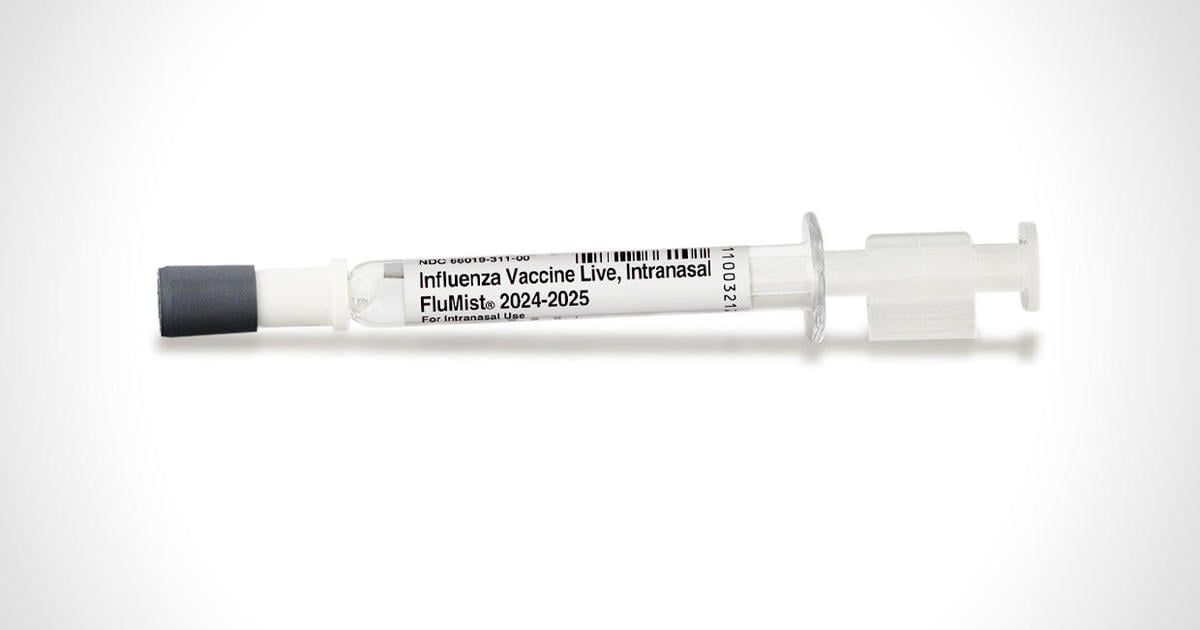
Nasal ones have been less effective in the past. Still better if it gets those who straight up won’t get anything if it’s a shot.
I think I’d rather have a sore arm than this crap in my nose and throat for however long.
+1 on preferring the needle, where I live it takes 15-30m to get the shot and it’s not very crowded.