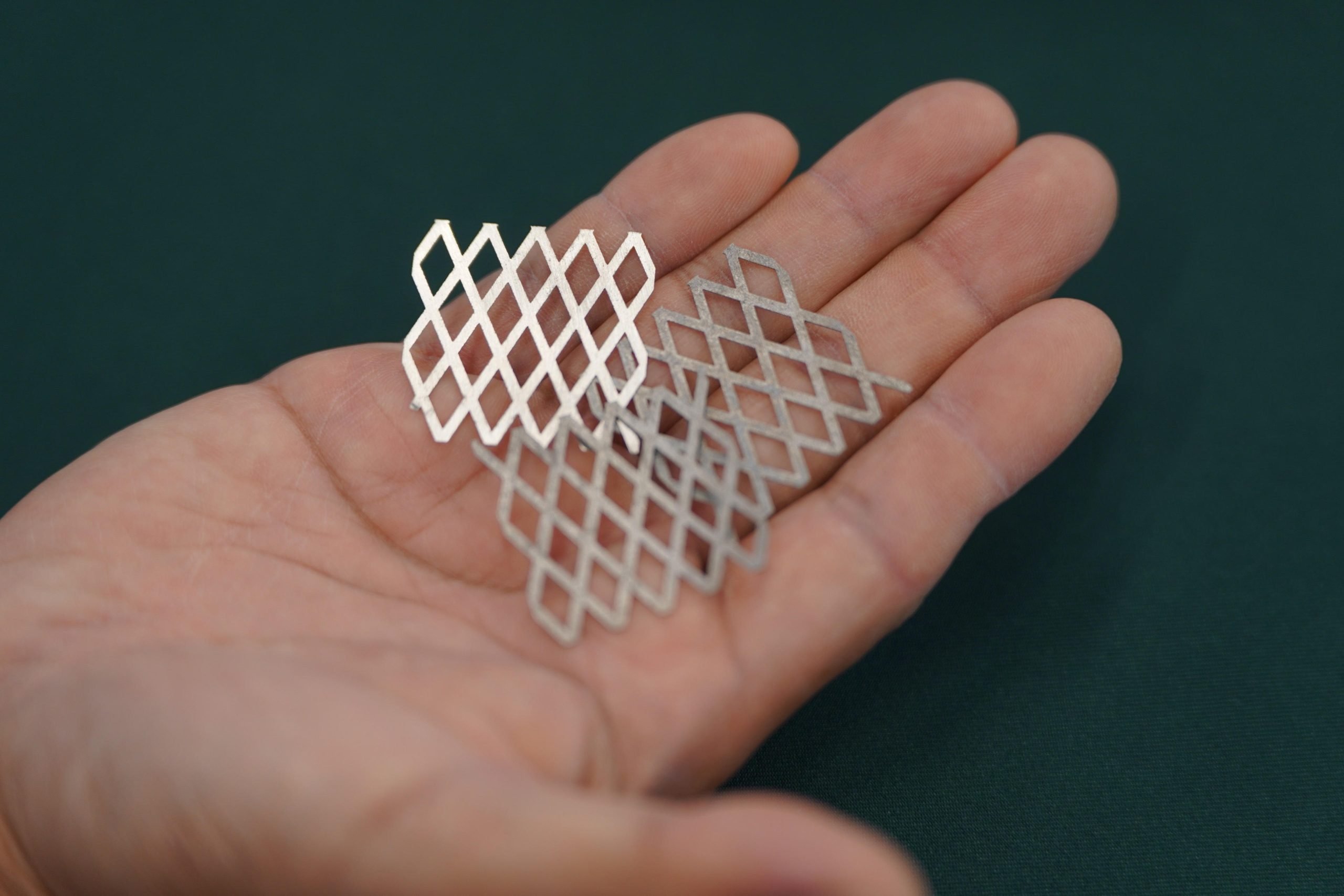
A process that normally lowers the corrosion resistance had the opposite effect when applied over an alternate known-good option. Chromium passivation is the known-good, manganese passivation is known to make it worse. Manganese-passivating chromium-passivated stainless looks like it’s way more corrosion resistant than either passivation on its own and non-passivated stainless
Also, daily reminder, stainless steel corrodes. It forms a corrosion layer that is hard and doesn’t change size compared to the base metal (unlike rust on normal steel, which expands), but it can still be prone to erosion particularly in oxygen-depleted water. Even 316.
I’m kinda glad stainless steel corrodes in the end. Else it would become a micro-fragment polluter like plastic.
I’d say a major difference is that steel is an alloy of natural inorganic elements. The components aren’t especially toxic, at least not any more than how they already exist in the environment. Microplastics are entirely man made and don’t have any natural, decent way to be broken down organically. Would you count sand as a pollutant? It’ll last way longer than plastic and we certainly ship it around everywhere. Eating sand is just part of marine life
How are the other properties? Will this stuff appear in knives? Hardness, edge retention, brittle… ness-ity?
Will this change my life, or is it just a curiosity to amuse material scientists?
This steels intended design use is hydrogen production through the electrolysis of salt water. Typically it is done with titanium because existing stainless steels corrode too much in the high chloride environment. But this novel process of adding corrosion resistance steel performs just as well as the titanium. It’s not a knife steel. As with most material science materials, this was designed for a specific use case in mind. Not all steels have to be good at everything. A knife super steel would probably be bad at hydrogen production for example.
Thanks!
True, steels are very specific, even within knife applications. For instance, there are certain steels used for knives for marine environments; they’re not usually used outside of those specialty knives because they give up other desireable characteristics for the corrosion resistance.
Which leads me back to why I asked the question. Corrosion/rust resistance is desireable in knives, and that’s what this sounded like. Ah, well… maybe it’ll inform the next generation of steels.
The way i read it is its another big step in the development of nanocomposites/meta materials.
You can sort of explain it by hand carving materials on a molecular level so they exibit all kinds of possibly exitic effects. Like deforming light or to kill certain germs.
Its one of my favorite tech to mention because the story of supposed normal looking but atomically weird materials found at ufo crashsites
brittle… ness-ity?
I love you!
And I love you, PlutoniumAcid!
You can’t explain that!
Of course I can’t. But they fucking should.
I was making a lame attempt at that Bill O’Reilly joke about magnets or whatever, but you’re right, they fucking should!
My favourite version of this meme, it lives rent free in my head.
That’s ancient internet wisdom.
Sage posting for real
Some people can, and I do not trust those people.
Probably because they reveal that fact to you?
It wasn’t even magnets, it was the tide.
And SS isn’t magnetic, either.
Explain that, juggalos.
300 series isn’t but 400 series is mildly magnetic
And that’s my lesson for you juggalos and juggalettes today - skelator.jpg
Checkmate atheists
I certainly can’t
Wouldja look at that?
That’s pretty NEAT!
NEATallurgy.
Who put it there?
deleted by creator
How do you know there’s ads if you didn’t click? 🤔
Also what kind of maniac doesn’t use an ad-blocker?
Maybe they are just traumatized from a past ad experience.
Might be my browser setup but I read through without seeing a single ad, even in the links at the bottom.
Use a browser with uBlock Origin
New steel? Want knife made from it. Now! Gimme!
Yay, now we can drain all the water from our overheated planet!
Yay, now we can use electrolysis on sea water to recombine and create fresh water easier!
Why is the solution to our problems always to tap into yet another finite resource? Can we not just stop buying shit we don’t need? Is it that hard to stop growing crops where they don’t belong? How about we stop paving over all our watersheds? Why’s the solution have to be “just start sucking it out of the ocean?”
Bro, what do you think happens to the water after it’s used?
It isn’t instantly converted back to water. Even if it were, it doesn’t instantly go back into the ocean. This over simplification of “hydrogen means free energy” is dangerous and is reminiscent of the early days of nuclear and fossil fuels. Don’t flame me for wanting to consider all the possible negative effects. Neglecting potential downsides is why our planet is in such a terrible state now.
Has anyone considered the effects of increasing the salt concentration of the ocean? And don’t tell me “the ocean is big, we couldn’t possibly have an effect” because that’s what climate deniers have been saying the about the atmosphere for decades. Our fresh water supplies were massive at one point too and look what we did to them. Our water needs are growing and will continue to grow. We need to consider and account for all potential side effects.
The increased salt concentration won’t be a problem because the melting glaciers will offset it.
Where do you think it drains to?
Calm down, joke police.