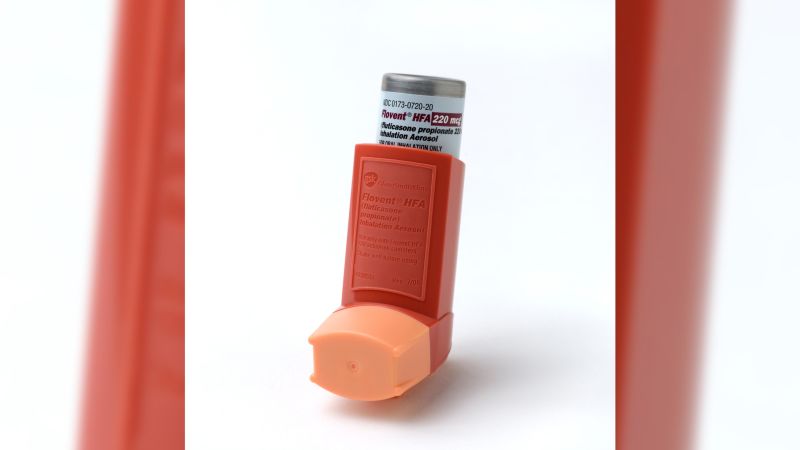
Starting January 1, a drug that thousands of patients depend on to help them breathe will disappear from pharmacy shelves, and doctors are concerned patients may have delays switching to alternatives and getting them covered by insurance.
Manufacturer GSK has said it’s discontinuing the branded asthma inhaler Flovent, and instead is making an “authorized generic” version, which is identical but without the same branding.
Physicians who treat patients with asthma say the authorized generic will work just as well as the branded drug, but it doesn’t appear to be covered as widely by insurers. That may mean patients will have to obtain new prescriptions and sort out coverage hurdles at the height of respiratory virus season.
“This medication has been the most commonly used inhaled medication for the past 25 or 30 years,” said Dr. Robyn Cohen, director of the Pediatric Pulmonary and Allergy Clinic at Boston Medical Center. “It’s the one that, overwhelmingly, pediatricians reach for when they decide that their patient needs a daily preventive medication. …The fact that it’s being discontinued is going to be a huge shock to the system for patients, for families and for doctors.”


Authorized generic is such a weird, profit-seeking term.
And as usual, the ultimate problem is not anything to do with the drug itself but the people trying to suck money out of it.
I was pretty confused by the article, but reading your comment made it make sense… I absolutely despise our Healthcare system.
I was like, how would a generic drug be harder to get and covered by less.